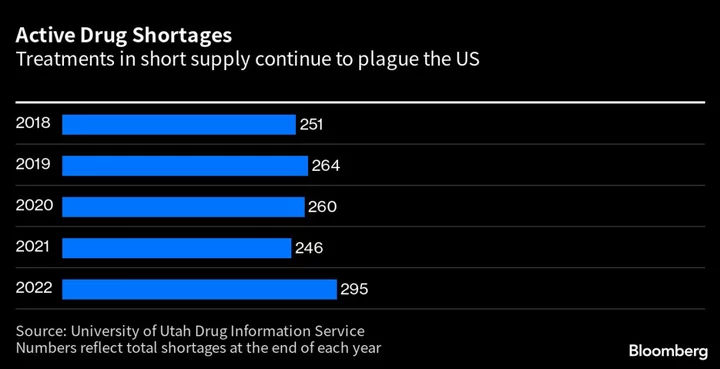
As US drug shortages hit a five-year high and concerns mount about the safety of medicines, the Biden administration has quietly assembled a team to address chronic problems hurting America’s drug supply.
Since the beginning of the year, a group of White House officials has been meeting frequently to increase the availability and quality of medications, according to several people familiar with the matter. The effort has intensified as Americans struggle to find common drugs like antibiotics and amid high-profile safety lapses like deadly eye drops.
The Biden administration first set its sights on drug shortages and quality issues in 2021, as part of an effort to bring manufacturing back to the US that includes other industries. But fixing the drug supply chain has been particularly challenging, prompting a more serious push.
President Joe Biden’s top domestic policy adviser, Susan Rice, has been a driving force behind the team, according to two of the people. The group is led by the Domestic Policy Council and National Economic Council.
Rice, who is leaving her post later this month, asked the group to finalize proposals by early spring with tangible ideas that the US government could execute, according to one of the people. The group missed that timeline because they have struggled to reach a consensus with the Food and Drug Administration, said five people familiar with the talks.
Most of the people interviewed for this article spoke on condition of anonymity. Asked for comment, a senior Biden administration official said the White House team’s work is a continuation of the administration’s focus on ensuring resilience of the nation’s pharmaceutical supply.
FDA Involvement
Two people familiar with the group’s efforts said the FDA has refused to fully answer questions and suggested that the agency lacks the funding or authority to make certain changes floated by the White House group.
An FDA official who spoke on the condition of anonymity said the agency has been cooperating with the White House but can’t fix the supply problems alone because larger market forces are driving generic drugmakers out of business, contributing to shortages. The companies that buy drugs in bulk pit manufacturers against each other and drive down prices, which shrinks drugmakers’ margins, the official said. This leaves manufacturers with little money to maintain robust supply chains or, in some cases, keep making drugs at all.
Another FDA official said the agency has suggested generic drugmakers need incentives such as tax breaks to boost US manufacturing.
“We remain committed to partnering across government, academia, and industry to strengthen and diversify the supply chain, further address drug shortages and ensure Americans continue to have access to drugs that are of high quality, safe and effective,” the FDA said in a statement.
Razor-Thin Margins
Generic drugs are cheap and have razor-thin margins, so manufacturers do everything they can to cut costs. The companies manufacture in facilities in lower-cost countries such as India and China that are subject to less scrutiny, where it’s easy for safety problems to develop, leading to recalls and shortages. And companies don’t make large excess quantities of drugs because they can’t afford to, leaving little room for unexpected spikes in demand.
To fix these problems, the White House group has met with a variety of players including Mark Cuban’s discount drug company, Cost Plus Drugs, the head of pharmacy at NYU Langone Health and Valisure, an independent testing lab in Connecticut, according to the people. The coalition is also in frequent communication with the Pentagon, the Department of Health and Human Services and the FDA, which HHS oversees.
Among the ideas the White House is looking at are: developing tools that could foresee potential drug shortages before they start, creating quality scores for manufacturing facilities, increasing the FDA’s unannounced inspections, robust testing of imported products, and requiring foreign manufacturers to keep electronic records instead of paper ones, since those can be destroyed.
After Bloomberg News reported details of the White House effort, the director of government affairs at Teva Pharmaceutical Industries Ltd., one of the largest generic drugmakers, wrote on social media that burdensome additional regulations would worsen shortages.
The White House team has met with other drug industry executives, three people familiar with the matter said. Teva’s Chief Executive Officer Richard Francis said Wednesday that he hasn’t met with the team but that he’s “pleased that the government is focusing on something as critical as access to medicines.”
The pharmaceutical supply chain is uniquely hard for the government to change in part because the US has taken a hands-off approach to drug manufacturing for so many years.
“There’s a lot of resistance in the industry to more transparency or anything that requires more cost in this low-margin business,” said Elisabeth Reynolds, a Massachusetts Institute of Technology lecturer and former special assistant to Biden for manufacturing and economic development at the National Economic Council. “We have to find ways to provide incentives to invest in quality, or put in place requirements to make them responsible for quality.”
The White House team is preparing to present leadership with solutions in coming weeks, according to a person familiar with the matter. A senior administration official said the group’s work will continue after Rice’s departure later this month.
Rice is being replaced by Neera Tanden, a senior adviser to Biden who formerly worked in the Obama and Clinton administrations.
(Updates with industry reaction from 14th paragraph.)
Author: Riley Griffin, Anna Edney and Ike Swetlitz