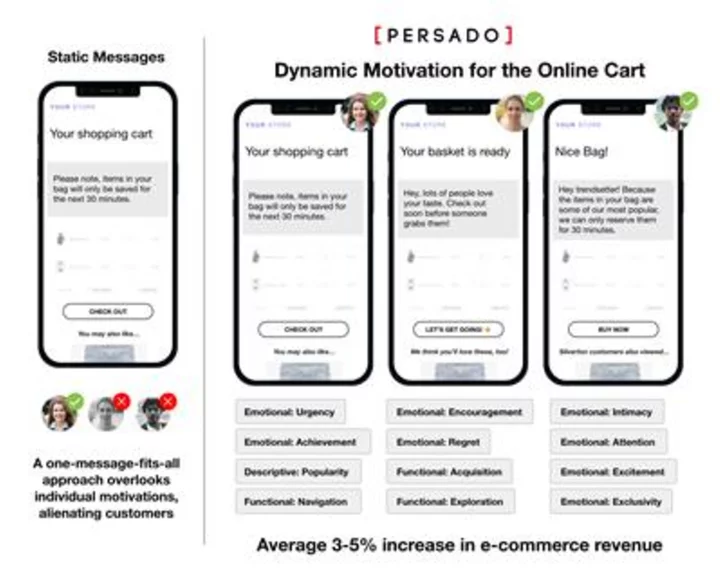
LEWISVILLE, Texas--(BUSINESS WIRE)--Jun 19, 2023--
Orthofix Medical Inc. (NASDAQ:OFIX), a leading global spine and orthopedics company, today announced the full commercial launch of the WaveForm ® A interbody for Anterior Lumbar Interbody Fusion (ALIF) procedures. The WaveForm A interbody seamlessly integrates with the company’s Meridian ALIF system for treating patients in need of fusion due to degenerative disc disease. The proprietary wave-like design of the WaveForm A interbody provides a balance of strength, porosity and stability, with a large implant graft aperture for bone graft material to aid in creating an osteoinductive environment to optimize supplemental fixation procedures.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20230619449429/en/
The 3D printed WaveForm A interbody for ALIF procedures features a proprietary wave-like design that provides a balance of strength, porosity and stability, with a large implant graft aperture for bone graft material. (Photo: Business Wire)
“Implant design and surface technology play a vital role in the bone growth process during fusion,” said Dr. Neil Arif Tayyab, orthopaedic spine surgeon at Girard Orthopaedic Surgeons, in San Diego, CA. “Newer designs such as the WaveForm A interbody can help stimulate a better bone growth response and give me greater confidence that the patient will have a successful fusion.”
The Meridian ALIF system is a modular instrument and implant system designed to help streamline ALIF procedures by providing diverse fixation options for single and multilevel ALIFs in a reduced number of trays. Meridian is compatible with both the WaveForm A interbody and the company’s Reef ™ A interbody featuring its proprietary NanoMetalene ® surface technology and Reef Topography ™. With the launch of WaveForm A, the Meridian system can more completely address the approximately $200 million dollar ALIF market segment in the U.S. 1
The 3D printed WaveForm A implants feature WaveForm ® technology, a repeating and continuous wave-like structure. WaveForm was created to withstand high compressive loads while delivering an endplate porosity that maximizes the potential for early stabilization 2,3. This balance of strength to porosity offers increased opportunity for bone packing, decreased stiffness profile and enhanced imaging properties. 3
“Our Meridian ALIF system integrates the most advanced technologies into our surgical sets, minimizing the need for multiple instrument sets during the procedure,” said Kevin Kenny, President of Global Spine. “The WaveForm A interbody is an exciting addition to our portfolio of solutions for ALIF procedures and enables us to provide a more streamlined option so surgeons can do more for their patients.”
Advancing Care with ALIF
The ALIF market can be segmented by material into non-3D printed metal, PEEK (standard and titanium-based advanced surface technologies), 3D printed metal, and machined bone markets. It is estimated that more than half of the U.S. ALIF interbody market today is addressed by PEEK devices. Due to their clinical advantages, 3D printed and advanced surface technologies-based interbody devices are expected to grow faster than the overall interbody market in upcoming years as they take market share from PEEK devices, ultimately leading to a larger market share than standard PEEK. 4
The WaveForm A interbody and the Meridian ALIF system are available in the U.S. Learn more about the Company’s interbody systems here.
1 Market research on file. Based off iData Research Inc. 2021 numbers.
2 Kelly, Cambre N., et al. "Design and structure–function characterization of 3D printed synthetic porous biomaterials for tissue engineering." Advanced healthcare materials 7.7 (2018): 1701095.
3 O. Al-Ketan, R. Rowshan, R.K. Abu Al-Rub, Topology-mechanical property relationship of 3D printed strut, skeletal, and sheet based periodic metallic cellular materials, Addit. Manuf. 19 (2018) 167-183.
4 Data on file.
About Orthofix
The newly merged Orthofix-SeaSpine organization is a leading global spine and orthopedics company with a comprehensive portfolio of biologics, innovative spinal hardware, bone growth therapies, specialized orthopedic solutions and a leading surgical navigation system. Its products are distributed in more than 68 countries worldwide.
The Company is headquartered in Lewisville, Texas and has primary offices in Carlsbad, CA, with a focus on spine and biologics product innovation and surgeon education, and Verona, Italy, with an emphasis on product innovation, production, and medical education for orthopedics. The combined company’s global R&D, commercial and manufacturing footprint also includes facilities and offices in Irvine, CA, Toronto, Canada, Sunnyvale, CA, Wayne, PA, Olive Branch, MS, Maidenhead, UK, Munich, Germany, Paris, France, and São Paulo, Brazil.
Forward-Looking Statements
This news release may include forward-looking statements within the meaning of Section 21E of the Securities Exchange Act of 1934, as amended, and Section 27A of the Securities Act of 1933, as amended. In some cases, you can identify forward-looking statements by terminology such as “may,” “will,” “should,” “expects,” “plans,” “anticipates,” “believes,” “estimates,” “projects,” “intends,” “predicts,” “potential,” “continue” or other comparable terminology. Orthofix cautions you that statements included in this news release that are not a description of historical facts are forward-looking statements that are based on the Company’s current expectations and assumptions. Each forward-looking statement contained in this news release is subject to risks and uncertainties that could cause actual results to differ materially from those expressed or implied by such statement. Applicable risks and uncertainties include, among others: the ability of newly launched products to perform as designed and intended and to meet the needs of surgeons and patients, including as a result of the lack of robust clinical validation; and the risks identified under the heading “Risk Factors” in Orthofix Medical Inc.’s Annual Report on Form 10-K for the fiscal year ended December 31, 2022, which was filed with the Securities and Exchange Commission (SEC) on March 6, 2023. The Company’s public filings with the Securities and Exchange Commission are available at www.sec.gov. You are cautioned not to place undue reliance on forward-looking statements, which speak only as of the date when made. Orthofix does not intend to revise or update any forward-looking statement set forth in this news release to reflect events or circumstances arising after the date hereof, except as may be required by law.
View source version on businesswire.com:https://www.businesswire.com/news/home/20230619449429/en/
CONTACT: Media Relations
Denise Landry
DeniseLandry@orthofix.com
214.937.2529Investor Relations
Louisa Smith, Gilmartin Group
IR@orthofix.com
KEYWORD: UNITED STATES NORTH AMERICA TEXAS
INDUSTRY KEYWORD: SURGERY MEDICAL DEVICES HOSPITALS BIOTECHNOLOGY OTHER HEALTH PHYSICAL THERAPY MANAGED CARE HEALTH GENERAL HEALTH
SOURCE: Orthofix Medical Inc.
Copyright Business Wire 2023.
PUB: 06/19/2023 07:00 AM/DISC: 06/19/2023 07:00 AM
http://www.businesswire.com/news/home/20230619449429/en