MONROE TOWNSHIP, N.J. & OXFORD, England--(BUSINESS WIRE)--Oct 2, 2023--
Blue Earth Diagnostics, a Bracco company and recognized leader in the development and commercialization of innovative positron emission tomography (PET) radiopharmaceuticals, today announced results from a post-hoc analysis from the Phase 3 SPOTLIGHT trial (NCT04186845) that investigated the use of POSLUMA® (flotufolastat F 18) PET in suspected biochemical recurrence of prostate cancer. The analysis examined the detection rate (% positive PET scans) in a subset of patients with low-very low Prostate Specific Antigen (PSA) levels. POSLUMA® (flotufolastat F 18) injection (formerly referred to as 18 F-rhPSMA-7.3) is indicated for positron emission tomography (PET) of prostate-specific membrane antigen (PSMA) positive lesions in men with prostate cancer with suspected metastasis who are candidates for initial definitive therapy or with suspected recurrence based on elevated serum prostate-specific antigen (PSA) level.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20230929119301/en/
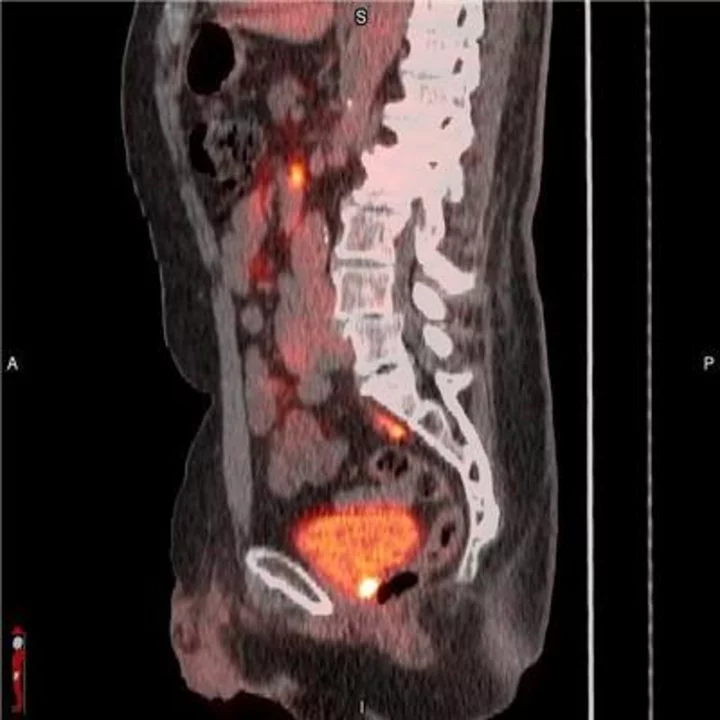
POSLUMA® (flotufolastat F 18) PET/CT image showing uptake in the prostate bed, consistent with recurrent prostate cancer Photo courtesy of Blue Earth Diagnostics
Results highlights:
- Overall, 68% (128/188) of evaluable patients with a PSA level of <1 ng/mL, 76% (51/67) of patients with a PSA of ≥0.5 – <1 ng/mL, and 64% (77/121) of patients with a PSA <0.5 ng/mL had a positive flotufolastat F 18 scan by majority read.
- Extrapelvic lesions were observed in 21% (25/121) of patients with a PSA <0.5 ng/mL, increasing to 39% (26/67) in patients with a PSA of ≥0.5 to 1 ng/mL.
“Recurrent prostate cancer presents clinical challenges, and the ability to determine the extent and location of recurrent disease is necessary to inform physicians and their patients for appropriate clinical management,” said Ashesh B. Jani, MD, MSEE, FASTRO, Winship Cancer Institute of Emory University, Atlanta, Ga., on behalf of the SPOTLIGHT Study Group. “The SPOTLIGHT study investigated the diagnostic performance of POSLUMA PET imaging as a potential decision-making aid in assessing suspected biochemical recurrence of the disease, and demonstrated precision diagnostic performance, with an overall 83% (322/389) detection rate. This post-hoc analysis further examined POSLUMA performance in 188 men with low-very low PSA levels. Results showed that more than two-thirds of these men were found to have positive POSLUMA scans, with a quarter of them having extrapelvic lesions. POSLUMA PET may be a useful tool for treatment planning, particularly in patients with suspected early recurrence of disease who may be candidates for curative salvage therapy.”
“We are pleased to present these results to the radiation oncology community at ASTRO,” said David E. Gauden, D.Phil., Chief Executive Officer of Blue Earth Diagnostics. “POSLUMA has recently been added to nationally recognized clinical oncology guidelines for prostate cancer, alongside and for all the same categories as the other currently FDA-approved PSMA PET radiopharmaceuticals. Our new product represents a new class of high-affinity PSMA-targeted radiopharmaceuticals based on novel radiohybrid technology and provides physicians with high-quality information based on these good detection rates at low PSA levels, high-affinity PSMA binding and low urinary bladder activity. The product is labeled with the radioisotope fluorine-18 ( 18 F) to leverage high image quality and to enable broad, readily available geographic access for patients via the manufacturing and distribution network of our commercial U.S. manufacturer and distributor, PETNET Solutions Inc, A Siemens Healthineers Company.”
The findings were discussed in an oral presentation at the 2023 ASTRO Annual Meeting on October 2, 2023, “ Detection Rate of 18 F-rhPSMA-7.3 PET in Patients with Suspected Prostate Cancer Recurrence at PSA Levels <1 ng/mL: Data from the Phase 3 SPOTLIGHT Study,” by Ashesh B. Jani, MD, MSEE, FASTRO, Winship Cancer Institute of Emory University, Atlanta, Ga., on behalf of the SPOTLIGHT Study Group. Full session details and the abstract are available in the ASTRO online program here.
About the study
The post-hoc analysis of SPOTLIGHT data determined flotufolastat F 18 detection rates (DR) at low-very low PSA levels. Patients enrolled in SPOTLIGHT underwent PET with scans evaluated by majority read of 3 blinded central readers. For the present analysis, all patients with an evaluable flotufolastat F 18 PET and a baseline PSA <1 ng/mL were selected. Overall (patient-level) and regional DR by majority read were determined, stratifying DR according to the patients’ baseline PSA level (<0.2, ≥0.2 – <0.3, ≥0.3 – <0.5, and ≥0.5 – <1 ng/mL).
- In total, 389 patients (median [range] PSA, 1.10 [0.03–135] ng/mL, 84 with intact prostate) had an evaluable flotufolastat F 18 scan. The overall DR was 83% (322/389) by majority read. Of the 389 patients with an evaluable flotufolastat F 18 scan, 188 had a baseline PSA <1 ng/mL and were eligible for the present analysis. Despite low patient numbers in some PSA categories, moderate to high DR were observed, with the patient-level DR shown to increase with increasing baseline PSA. Overall, 68% (128/188) of patients with a PSA <1 ng/mL and 64% (77/121) of patients with a PSA <0.5 ng/mL had a positive flotufolastat F 18 scan by majority read. Regional DRs were broadly consistent across all PSA categories. Extrapelvic lesions were observed in 21% (25/121) of patients with a PSA <0.5 ng/mL, increasing to 39% (26/67) in patients with a PSA of ≥0.5 to 1 ng/mL.
- No serious adverse reactions were attributed to flotufolastat F 18 in the SPOTLIGHT study. Overall, 16 (4.1%) patients had at least one treatment-emergent adverse event that was considered possibly related/related to flotufolastat F 18. The most frequently reported events were: hypertension: 1.8% (n=7); diarrhea: 1.0% (n=4); injection site reaction: 0.5% (n=2), and headache: 0.5% (n=2).
About Radiohybrid Prostate-Specific Membrane Antigen (rhPSMA)
Radiohybrid Prostate-Specific Membrane Antigen (rhPSMA) compounds consist of a radiohybrid (“rh”) Prostate-Specific Membrane Antigen-targeted receptor ligand which attaches to and is internalized by prostate cancer cells, and they may be radiolabeled with imaging isotopes for PET imaging, or with therapeutic isotopes for therapeutic use – providing the potential for creating a true theranostic technology. Radiohybrid technology and rhPSMA originated from the Technical University of Munich, Germany. Blue Earth Diagnostics acquired exclusive, worldwide rights to rhPSMA diagnostic imaging technology from Scintomics GmbH in 2018, and therapeutic rights in 2020, and sublicensed the therapeutic application to its sister company Blue Earth Therapeutics. Blue Earth Diagnostics received U.S. Food and Drug Administration approval for its radiohybrid PET diagnostic imaging product for use in prostate cancer in 2023. rhPSMA compounds for potential therapeutic use are investigational and have not received regulatory approval.
Indication and Important Safety Information About POSLUMA
INDICATION
POSLUMA® (flotufolastat F 18) injection is indicated for positron emission tomography (PET) of prostate-specific membrane antigen (PSMA) positive lesions in men with prostate cancer
- with suspected metastasis who are candidates for initial definitive therapy
- with suspected recurrence based on elevated serum prostate-specific antigen (PSA) level
IMPORTANT SAFETY INFORMATION
- Image interpretation errors can occur with POSLUMA PET. A negative image does not rule out the presence of prostate cancer and a positive image does not confirm the presence of prostate cancer. The performance of POSLUMA for imaging metastatic pelvic lymph nodes in patients prior to initial definitive therapy seems to be affected by serum PSA levels and risk grouping. The performance of POSLUMA for imaging patients with biochemical evidence of recurrence of prostate cancer seems to be affected by serum PSA levels. Flotufolastat F 18 uptake is not specific for prostate cancer and may occur in other types of cancer, in non-malignant processes, and in normal tissues. Clinical correlation, which may include histopathological evaluation, is recommended.
- Risk of Image Misinterpretation in Patients with Suspected Prostate Cancer Recurrence: The interpretation of POSLUMA PET may differ depending on imaging readers, particularly in the prostate/prostate bed region. Because of the associated risk of false positive interpretation, consider multidisciplinary consultation and histopathological confirmation when clinical decision-making hinges on flotufolastat F 18 uptake only in the prostate/prostate bed region or only on uptake interpreted as borderline.
- POSLUMA use contributes to a patient’s overall long-term cumulative radiation exposure. Long-term cumulative radiation exposure is associated with an increased risk for cancer. Advise patients to hydrate before and after administration and to void frequently after administration. Ensure safe handling to minimize radiation exposure to the patient and health care providers.
- The adverse reactions reported in ≥0.4% of patients in clinical studies were diarrhea, blood pressure increase and injection site pain.
- Drug Interactions: androgen deprivation therapy (ADT) and other therapies targeting the androgen pathway, such as androgen receptor antagonists, may result in changes in uptake of flotufolastat F 18 in prostate cancer. The effect of these therapies on performance of POSLUMA PET has not been established.
To report suspected adverse reactions to POSLUMA, call 1-844-POSLUMA (1-844-767-5862) or contact FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Full POSLUMA prescribing information is available atwww.posluma.com/prescribing-information.pdf.
About Blue Earth Diagnostics
Blue Earth Diagnostics, an indirect subsidiary of Bracco Imaging S.p.A., is a growing international molecular imaging company focused on delivering innovative, well-differentiated diagnostic solutions that inform patient care. Formed in 2014, the Company’s success is driven by its management expertise and supported by a demonstrated track record of rapid development and commercialization of positron emission tomography (PET) radiopharmaceuticals. Blue Earth Diagnostics’ expanding oncology portfolio encompasses a variety of disease states, including prostate cancer and neuro-oncology. Blue Earth Diagnostics is committed to the timely development and commercialization of precision radiopharmaceuticals for potential use in imaging and therapy. For more information, please visit: www.blueearthdiagnostics.com.
About Bracco Imaging
Bracco Imaging S.p.A., part of the Bracco Group, is a world-leading diagnostic imaging provider. Headquartered in Milan, Italy, Bracco Imaging develops, manufactures and markets diagnostic imaging agents and solutions. It offers a product and solution portfolio for all key diagnostic imaging modalities: X-ray imaging (including Computed Tomography-CT, Interventional Radiology, and Cardiac Catheterization), Magnetic Resonance Imaging (MRI), Contrast Enhanced Ultrasound (CEUS), and Nuclear Medicine through radioactive tracers and novel PET imaging agents to inform clinical management and guide care for cancer patients in areas of unmet medical need. Our continually evolving portfolio is completed by a range of medical devices, advanced administration systems and dose-management software. In 2019 Bracco Imaging enriched its product portfolio by expanding the range of oncology nuclear imaging solutions in the urology segment and other specialties with the acquisition of Blue Earth Diagnostics. In 2021, Bracco Imaging established Blue Earth Therapeutics as a separate, cutting-edge biotechnology vehicle to develop radiopharmaceutical therapies. Visit: www.braccoimaging.com.
View source version on businesswire.com:https://www.businesswire.com/news/home/20230929119301/en/
CONTACT: For Blue Earth Diagnostics (U.S.)
Priscilla Harlan
Vice President, Corporate Communications
(M) (781) 799-7917
priscilla.harlan@blueearthdx.comFor Blue Earth Diagnostics (UK)
Clare Gidley
Associate Director Marketing and Communications
Tel: +44 (0) 7917 536939
clare.gidley@blueearthdx.comMedia
Sam Brown Inc.
Mike Beyer
(M) (312) 961-2502
mikebeyer@sambrown.com
KEYWORD: EUROPE UNITED STATES UNITED KINGDOM NORTH AMERICA NEW JERSEY
INDUSTRY KEYWORD: RESEARCH CLINICAL TRIALS BIOTECHNOLOGY RADIOLOGY HEALTH PHARMACEUTICAL OTHER SCIENCE SCIENCE ONCOLOGY
SOURCE: Blue Earth Diagnostics
Copyright Business Wire 2023.
PUB: 10/02/2023 06:30 PM/DISC: 10/02/2023 06:29 PM
http://www.businesswire.com/news/home/20230929119301/en